Studying the human body to drive scientific progress
François R. sits comfortably in front of a computer monitor, a look of intense concentration on his face. An instruction flashes up on the screen: “Hand to mouth with grasp.” He slowly brings his fingers toward his lips. But for the 70-year-old from Valais, this is harder than it sounds: in August 2019, he suffered a stroke that left him paralyzed on his right side from the shoulder down. The electroencephalogram (EEG) cap on his head carries signals from his brain down dozens of wires to a computer, where an algorithm translates sheer willpower into instructions for the robotic glove on his hand. He is unlikely to make a full recovery any time soon. But he’s here performing these tasks, under the watchful eye of a scientist, for a different reason: to advance research. “We’re trialing this system as a potential therapeutic intervention to improve motor-function recovery for stroke survivors,” says Meltem Oflar, a technical specialist at EPFL’s Hummel Lab.
For the past two years, François R. has been involved in various research programs in the laboratory specialized in translational neuroscience and neurological disorders (including stroke), located at the Clinique Romande de Réadaptation (CRR) in Sion. “This is good for me and doesn’t hurt,” he says. “There are no downsides. The stroke left me paralyzed down my right side and unable to talk. I had a choice: give up or fight on. Coming to the Suva clinic to take part in this research has really helped me cope.”
Recruiting volunteers
Each year in Switzerland, around 16,000 people suffer a stroke – a serious medical condition that kills one in five victims. Stroke is the third-leading cause of death nationally, after cardiovascular diseases and cancers. Moreover, only 15–20% of survivors make a full recovery, with most victims experiencing some degree of lasting motor or cognitive impairment.
Scientists are learning more about factors that make someone more likely to survive a stroke, including age, genetics, blood pressure, smoking history and cholesterol levels. But, as is often the case with brain disorders, little is still known about the recovery process in severe stroke victims. At the Hummel Lab, researchers are exploring how post-stroke impairments develop and what markers could predict a patient’s progress in the short, medium and long term. “Some patients recover naturally following a severe stroke, whereas others don’t,” explains Prof. Friedhelm Hummel. “The assumption is that patients in different groups need different therapies – what we call personalized medicine – and that treatment intensity is a key factor in the recovery process. We are dimensions away of what should be done.”
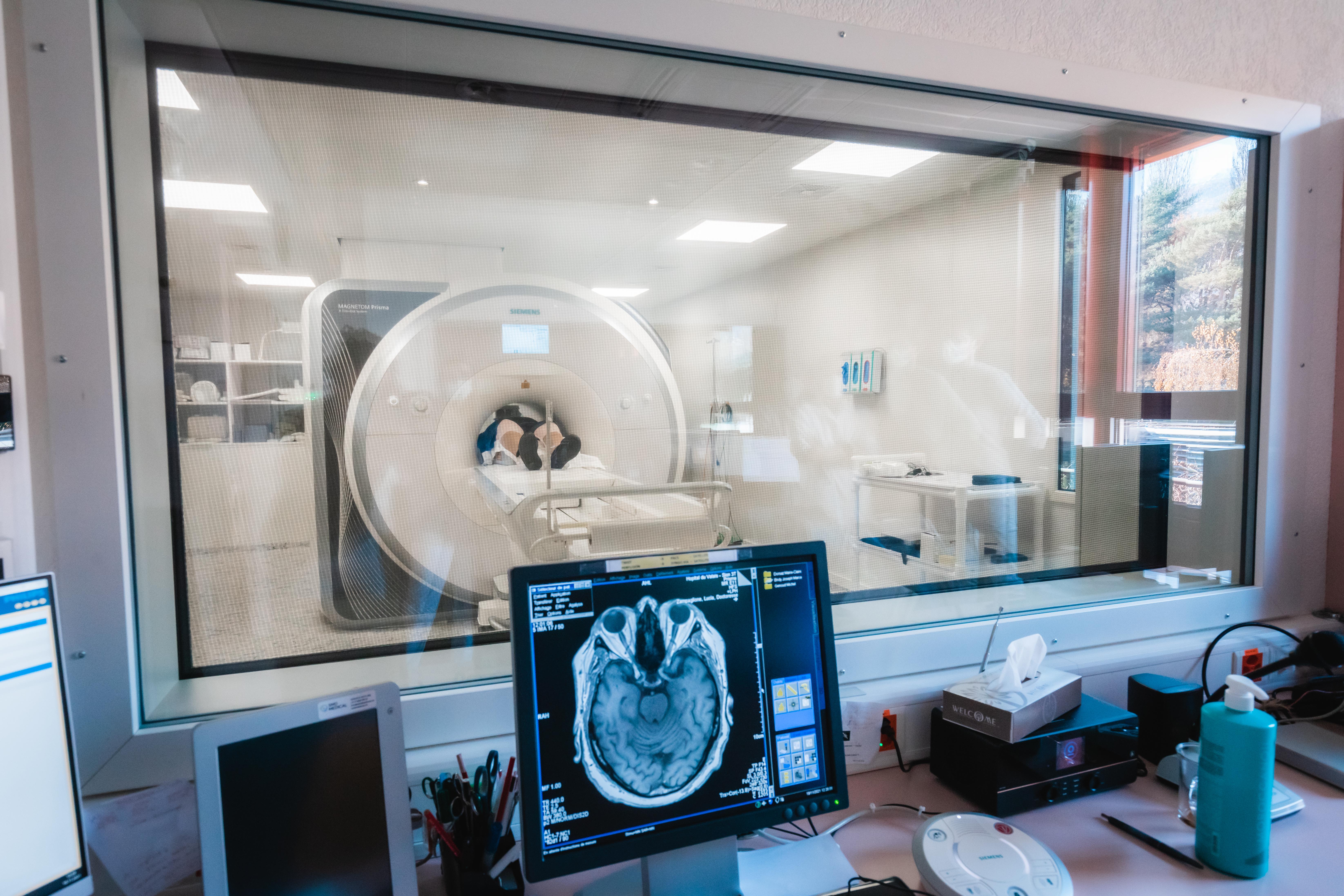
To study the brain, the Hummel Laboratory at EPFL uses an MRI scanner, which makes it possible to observe the interactions between different neuronal networks. © Jamani Caillet, EPFL 2021
Understanding the underlying processes and developing therapies involves both fundamental and clinical research. The Hummel Lab has a valuable working relationship with medical professionals, recruiting most patients for its programs from Valais Romand Hospital Center (CHVR) and the CRR clinic in Sion.
The team at the lab has special access to patients at both facilities: the first provides acute-phase treatment at its stroke unit, while the second specializes in rehabilitative therapy. The researchers discuss and review the inclusion and exclusion criteria with the medical team before approaching eligible patients. “We talk to the patients in person, or with relatives, explaining what we want to do, why and for what purpose, and what they can expect,” says Hummel. “They then decide whether or not they want to take part.” This is how the team recruited François R., who was approached soon after he was admitted to Valais Hospital (HVS) in Sion. “I didn’t agree right away,” he says. “But I soon changed my mind when I realized the value of the work they were doing. They told me I might see some improvement in my own condition, but that, more importantly, by taking part I’d be benefiting future stroke victims. That’s why I decided to sign up. I wanted to help.”
Strict licensing process
Like many EPFL programs, the studies carried out by the team at the Hummel Lab are classed as clinical trials – a type of research that often follows pre-clinical trials involving computer models, cell cultures or animal models. The purpose of these trials is to evaluate a therapeutic intervention or to learn more about how the human body functions under experimentally controlled conditions (in the quest for innovative treatments, for example). This kind of research can only be performed with human participants if equivalent results cannot be obtained by other means. Clinical trials are subject to a strict licensing process: researchers have to apply to the relevant Cantonal Ethics Committee – which is part of the swissethics umbrella organization – for approval. The committee only gives the green light if the benefit to participants outweighs the risk.
Under the Swiss Constitution, individuals can only take part in scientific research on a voluntary basis and must give their informed consent. Swiss law also protects the dignity and privacy of human trial participants.
“For our research into acute conditions, most patients we approach agree to participate”, explains Hummel. “But they’ve just had a stroke, so they have other things weighing on their mind. Even so, we’ve managed to recruit 80 volunteers for a trial we started two years ago.”
“I agreed without hesitation”
In a room adjoining François R.’s, insulated to prevent electromagnetic interference, a female patient is taking part in another Hummel Lab program known as Towards Individualized MEdicine in Stroke (TiMeS), which investigates changes in brain function following a stroke. She also has an EEG cap on her head but, unlike François R., she isn’t wearing a robot glove: in this study, patients have to remain perfectly still. On the monitor is a series of lines, each recording spontaneous brain activity from one of the 64 electrodes in the cap. “Oh boy!” she exclaims when she sees the amount of "noise" caused on the lines after a simple blink.

A scientist from the Hummel Lab stimulates the motor cortex of his patient to create a muscle contraction in the forearm. This allows to record the response of the muscles at all the electrodes.© Jamani Caillet, EPFL 2021
Volunteers undergo tests one week, three weeks, three months and one year after suffering a stroke. The team uses the results to monitor how well the patient is progressing, to identify the underlying recovery mechanisms, and to detect markers that might serve as predictors of recovery. As an observational study, TiMeS marks the first step in a long-term research program. As such, developing actual therapies is not its purpose. But that fact didn’t deter this particular patient, who was also approached to take part while she was recovering at the Sion hospital.
“I agreed without hesitation,” she says. “But they gave me some literature to read and 24 hours to come to a decision all the same. In my view, it’s a worthwhile cause, especially if by taking part I can help other people who haven’t recovered as well as I have. After waking up, I was more concerned about the state I was in than by the sight of a scientist coming to see me: I couldn’t drink anything on that first day and I was struggling to speak. If someone had told me they could do something to help me, I would have been delighted. I probably won’t benefit personally from this research when the study is over. But strokes can affect anyone without warning. That’s why scientific progress is so important.”
