Methods for bypassing and treating spinal cord injury
A video of a Parkinson’s patient went viral in early November. It showed how Marc, 62, was able to walk almost normally again – rather than “freezing” at every doorstep – thanks to a neural implant.
A few months earlier, another video showed how a man who’d been left paralyzed by an accident regained the ability to move his legs again using only his thought commands. The secret in both these cases is a system of flexible electrodes implanted directly on the patients’ spinal cords. These implants relay brain signals across the injured area and on to the patients’ limbs.

A neuroprosthetic has allowed Marc, a first patient with Parkinson’s, to be treated, enabling him to walk comfortably, confidently and without falling. © Gilles Weber / CHUV
In-depth understanding
Courtine began studying the brain signals that enable us to walk as part of his PhD thesis in 2003. He then spent years conducting extensive research in order to describe the kinds of electric signals that are sent down the spinal cord and how they’re arranged. In 2012, Courtine’s discoveries led to an amazing achievement: a protocol that allowed paraplegic rats to walk again. This breakthrough, published in Science that same year, was a springboard for Courtine and his team. “The first step in our research was to obtain an in-depth understanding of the physiological mechanisms behind the act of walking,” he says. “Only then did we start developing electrostimulation procedures that could replicate the signals sent by the brain.”
Courtine’s success is also due to the experts he works with. One of them is Stéphanie Lacour, a fellow EPFL professor who’s specialized in flexible implants – the electrodes used in Courtine’s system were developed in her lab. They’re placed directly on the dura mater in order to simulate the brain signals that can’t be transmitted naturally because of a spinal cord injury. The feat of implanting the electrodes was accomplished by Jocelyne Bloch, a neurosurgeon at Lausanne University Hospital (CHUV).

Work on spinal cord regeneration began in 2012 using rats. Photo: EPFL - CC-BY-SA 4.0
A large number of possible indications
This sort of “bridge” between the brain and limbs could be useful for treating a variety of afflictions, not just spinal cord injuries. People with Parkinson’s disease, for example, also have trouble with motor function. And individuals suffering from multiple system atrophy (MSA) – where dangerous drops in blood pressure mean patients are often bedridden – can benefit from targeted spinal cord stimulation to restore normal blood pressure. “What’s great about our work is that it can be applied to a wide range of pathologies and neurological disorders,” says Courtine.
His research team also has the advantage of being able to draw on the latest technology. For instance, they used artificial intelligence to interpret and then replicate the brain signals needed to trigger the right movements in a patient’s limbs.
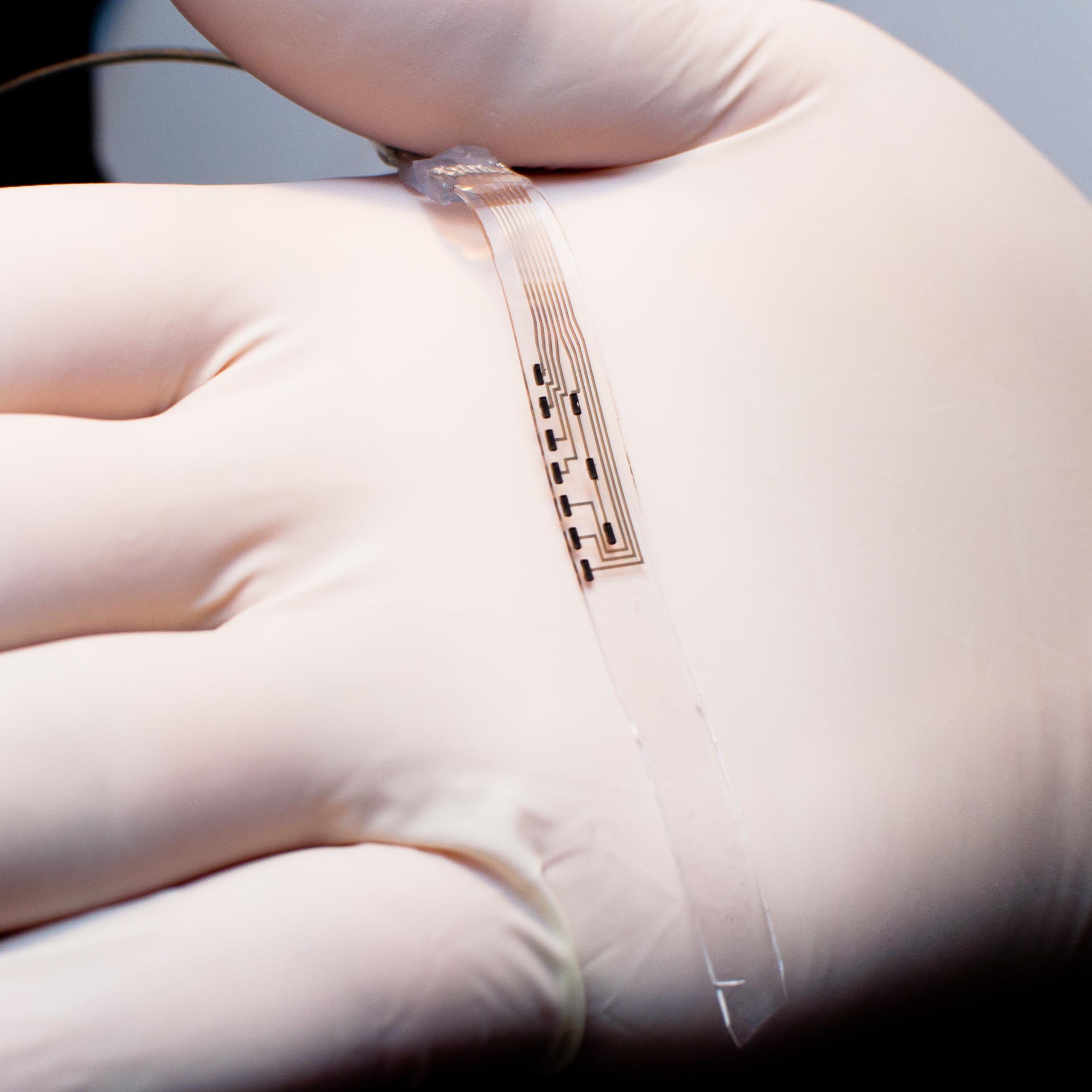
The development of flexible electrodes has led to the first clinical applications in paraplegic patients and then in a Parkinson's patient. Photo: EPFL - CC-BY-SA 4.0
Developing a strategy for healing spinal cords
Together, Courtine and Bloch head the .NeuroRestore center based jointly at EPFL and the CHUV. Their research there goes beyond developing implants: they’re also looking at how nerve fibers can be regenerated across spinal cord injuries through a combination of gene therapies and rehabilitation procedures potentially involving neural prostheses. “We believe that, in the future, doctors will be able to fully treat spinal cord injuries by applying two parallel approaches – a biological one to stimulate nerve-fiber regrowth, and a neuroprosthetic one in which our implants restore effective communication between the brain and the neurons controlling motor function,” said Courtine in September, commenting on a recently published study in Science, which he co-authored, that describes a strategy for regrowing nerve fibers in mice.
While the results obtained by Courtine and his team are remarkable, for now they have helped only a small number of patients taking part in clinical trials. But the scientists have their sights set high. Thanks to the work they’re doing through both .NeuroRestore and their startup Onward Medical, these breakthroughs will likely be only the first steps in the development of therapeutic approaches that will improve the lives of many.
