How bacterial cords spread tuberculosis
Tuberculosis is a lung disease caused by the Mycobacterium tuberculosis bacterium (Mtb). According to the WHO, tuberculosis afflicts 10 million people globally and claims 1.5 million lives. It is the leading cause of death among HIV patients, and a major contributor to antimicrobial resistance. Astonishingly, a quarter of the global population is estimated to harbor Mtb but not develop the disease.
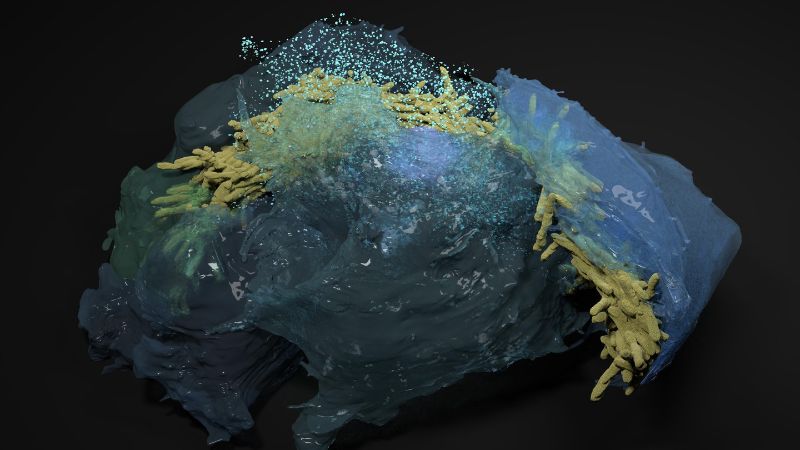
Bacterial cords
One of the peculiarities of Mtb, discovered in the 1940’s, is that it grows into “cords” inside the cells it infects – especially the alveolar cells of the lungs that handle oxygen exchange in the body. The Mtb cords are actually biofilms, characteristic structures that many bacteria species form to survive and proliferate (a well-known example is tooth plaque).
The Mtb cords grow within infected cells, and are connected to the bacterium’s virulence and the persistence of the infection. Understanding the mechanics of how they work is critical for devising strategies to combat tuberculosis more effectively.
Untangling the cords
A new study led by researchers at EPFL now sheds light on the formation and function of Mtb cords in alveolar cells. The findings significantly increase our understanding of tuberculosis pathology and could help develop novel treatment strategies. Published in Cell, the study was led by Vivek Thacker in the group of John McKinney at EPFL’s School of Life Sciences.
The researchers looked at the mechanopathology of Mtb cords. To this, they used an advanced a microengineered biochip called “lung-on-a-chip”, which mimics the structure and function of human lung tissues. Combined with mouse models, the team was able to mimic the early stages of TB infection and get a closer look at how individual Mtb bacteria grow into cords within alveolar cells in the lungs.
The approach revealed five major aspects of Mtb cords. First, that they are “high-aspect-ratio” structures, which means that their length is considerably longer than their width: they are long, thin threads. This shape is essential for understanding the mechanopathology and behavior of cords within host cells during tuberculosis infection.
Second, the cords are structurally resilient and maintain their integrity against mechanical perturbations due to a “phase transition” – a change in the state of the lipid monolayers of Mtb when they are compressed, which enables it to store mechanical energy. The scientists determined this through “agent-based” simulations, a computational approach that showed how this mechanical energy storage facilitates the formation and maintenance of the cord structures.
Third, the tightly packed Mtb bacteria within the cords can evade antibiotic clearance, showing how the efficiency of antibiotic treatments for tuberculosis can be limited.
Fourth, and very importantly, the cords literally “squeeze” the nucleus of the host cell, which in turn impairs the cell’s immune responses. This is a crucial finding, as it helps explain how Mtb suppresses host defenses, making the infection hard to clear.
Fifth, the cords can penetrate through and between cells, which makes it easy for Mtb to spread to new tissues, and further complicate the host’s ability to fight off the infection. In fact, the tightly packed Mtb bacteria could regrow in cords even after exposure to clinically relevant antibiotic concentrations. This finding is especially concerning as it suggests that current antibiotic treatments might be less effective due to reduced penetration to individual bacteria within these cord structures.
The study adds a new dimension to our understanding of tuberculosis infection, and potentially its treatment. “We provide a conceptual framework for the biophysics and function in tuberculosis infection and therapy of cord architectures independent of mechanisms ascribed to single bacteria” says Vivek Thacker. “By thinking of Mtb in infection as aggregates and not as single bacteria, we can imagine new interactions with host proteins for known effectors of Mtb pathogenesis, and a new paradigm in pathogenesis where forces from bacterial architectures affect host function.”