The wondrous world of light antennas
Without them, the entire world would always be black for us. The fact that we can look at the blue sky, read a book, or watch an action film on television we owe entirely to the photoreceptors: proteins that react to light.
Such proteins provide indispensable services to all life forms: single-celled algae, for example, use them to sense what direction it pays to swim in; plants can turn towards the sun thanks to these molecules. Many animals and humans, in turn, pick up light with highly developed organs, the eyes, and process the signals into complex impressions in the brain. Such light receptors also reset our internal clocks every day.
The principle is always the same: the proteins are integrated into the membranes – fatty envelopes around the cells – and convert light into a biological signal. The first and essential step of their activation is to flip a switch from off to on. But how exactly does the energy stored in a beam of light bring about the changes in photoreceptors that stand at the start of all further reactions? "So far, nobody has got to the bottom of this fundamental question," explains Gebhard Schertler, head of PSI’s Biology and Chemistry Division, who has been investigating proteins of this type for more than 30 years. To solve this and other mysteries, PSI researchers are studying the structure of light-sensitive proteins and their dynamic transformations.
How a cat lands on its feet
The off-to-on switching processes that go on constantly in our eyes are incredibly fast, taking only quadrillionths of a second. To probe these lightning-like processes in depth, very special research facilities are required, such as the X-ray free-electron laser SwissFEL, which was inaugurated at the end of 2016 and is thus the newest of the large research facilities at PSI. "With SwissFEL, we are taking structural biology to the next level," explains Jörg Standfuss, a scientist in the PSI Laboratory for Biomolecular Research. SwissFEL enables researchers to capture something like an ultrahigh-resolution film of biochemical processes in order to study a process down to the last detail – no matter how fast it is. "In this way we really can fully and completely understand how these light-sensitive proteins work."
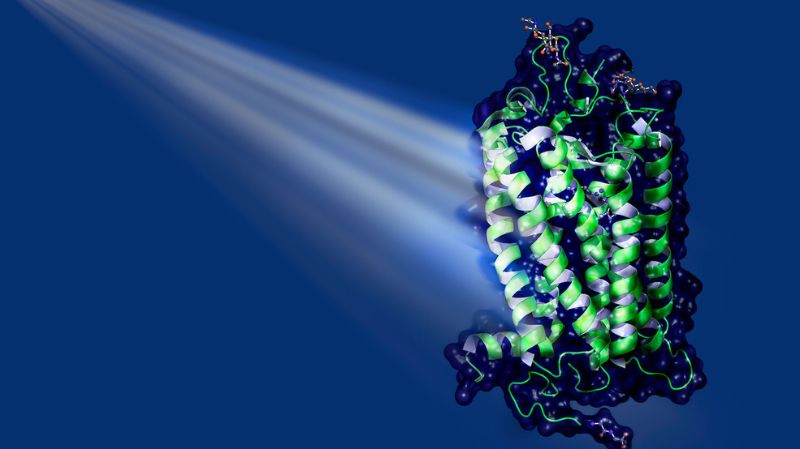
One of the most important natural light receptors in humans and animals is the rhodopsin family. In the human eye, these proteins are part of the rod cells, the sensory cells that specialise in the perception of light and dark. Fixed in the middle of rhodopsins is a small elongated molecule: retinal, a derivative of vitamin A. When light hits retinal, the molecule absorbs the energy and, as a result, is transformed (see illustration on the left). Retinal changes its three-dimensional shape. This in turn leads to structural changes in the protein, which now can bind to other proteins in the cell, the socalled G proteins. This sets in motion a cascade of biochemical and biophysical processes. The result might be, for example, the perception of a flash of light in the brain.
Valérie Panneels, a scientist in the PSI Laboratory for Biomolecular Research, wants to understand exactly how the structural change of the retinal takes place inside the protein. "Let’s compare retinal with a cat that falls back-first from a tree and lands on its feet. The question is: What states does the cat adopt during its fall, that is, while it is trying to right itself?" Even with cats, the process is so quick that it cannot be seen with the naked eye. All the more so in the case of retinal: intermediate states only exist for a few quadrillionths of a second.
Panneels knows that the retinal cat turns its shoulder first and then its stomach. But it’s still a big mystery why the transformation of retinal takes place so efficiently in the eye. "It’s one of the fastest and most directed reactions that occur in nature," says Valérie Panneels. The reaction is only this efficient, however, when the molecule is bound in the protein, not if it is floating freely in solution.
So the protein exerts a strong influence on the direction of the reaction – but exactly how is not clear to science. "If this question could be answered, the construction of SwissFEL would already have been worth it," says Standfuss. This knowledge would yield numerous new possibilities for further research and applications in medicine and biology.
Pumping instead of seeing
Evolution has also shaped light-sensitive proteins for a purpose other than seeing: they made it possible, for the first time, for living things to obtain energy from sunlight. Many bacteria and single-celled algae, for example, possess light-driven pumps in the cell membrane. These are proteins that, when exposed to light, change their shape in such a way as to transport ions – that is, small charged particles – out of the cell or into it. This enables the single-celled organisms to adjust themselves to the pH value, salinity, and other characteristics of their environment.
One such light-driven pump is bacteriorhodopsin. This protein transports protons and is found, for instance, in so-called halobacteria, a group of unicellular microorganisms that thrive in extremely salty lakes. Even though this single-celled organism is not closely related to humans biologically, bacteriorhodopsin is not so different from human rhodopsin: it too binds retinal and changes its shape when exposed to light. It does not, however, bind to G proteins, and – despite the name it was given historically – it belongs to a different class of proteins, the microbial opsins.
In 2016, Jörg Standfuss made, for the first time, a film of the pumping process of bacteriorhodopsin. Then in 2020, his group captured the pumping process of another light-driven pump in action: the sodium pump of a marine bacterium.
"Thanks to SwissFEL, PSI is a world leader in research on rhodopsins and their structural dynamics," says Przemyslaw Nogly, once a postdoc with Standfuss and now head of his own research group at ETH Zurich. With SwissFEL, Nogly is investigating the chloride pump halorhodopsin, which in halobacteria transports chloride ions into the cell from the outside. "We are particularly fascinated by the question of how the energy of the absorbed light is used to drive the transport of chloride," explains Nogly. In the meantime, SwissFEL is contributing to the solution of this mystery as well.
On and off
Understanding natural light antennas advances not only basic research, but also so-called optogenetics. With this technology, researchers are trying to build light-sensitive proteins as tiny switches in animal or human cells. Processes inside the target cells could then be switched on and off simply by irradiating them with light. Hopefully this could provide the basis for a broader understanding of biological processes in our body and pave the way for new therapies.
In the early 2000s, for the first time, researchers in the USA and Europe introduced a light receptor into nerve cells in a targeted way to control their activity (see infographic on page 16). This was the channel protein channelrhodopsin from a freshwater alga. The genetic material for this channel was introduced into the nerve cells of rats so that these cells would produce the ion channel in a petri dish. The channelrhodopsin opened when irradiated with blue light and let positively charged ions flow into the cells, whereupon the cells were activated. By means of such newly introduced ion channels, nerve cells can be controlled in real time with light from the outside.
All previously developed optogenetic tools have one major disadvantage, explains Valérie Panneels: they are applicable almost exclusively in nerve cells. "We are now working on developing optogenetic proteins that can also be used in other cells and for other functions – in virtually every organ." This would drastically expand the possibilities for applications of this technology.
The ideal target is the family of receptors that also includes rhodopsin: so-called G-protein-coupled receptors, GPCRs for short. They can be found in almost every cell in our body; they mediate numerous functions, from the senses of smell and taste, to regulating the heart rate, to initiating an inflammatory reaction. Therefore GPCRs are also extremely important targets for active ingredients in medicine. It is estimated that more than onethird of all currently approved drugs work by acting on this family of proteins.
Big plans
In the coming years Gebhard Schertler, working as part of a Swiss-European research team, wants to lay the foundations for developing light-controllable switches that can be used universally (see infographic on page 16). The consortium includes Peter Hegemann from the Humboldt University in Berlin, Sonja Kleinlogel from the University of Bern, and Rob Lucas from the University of Manchester in the United Kingdom.
The team wants to create so-called chimeric proteins, which are made up of two parts: a light-sensitive head that can be switched on and off by light, and a body that, when activated, switches on a very specific process in the cell.
However, rhodopsin from vertebrates, including humans, is not suitable for tiny switches like this, explains Gebhard Schertler. "Every time a rhodopsin is activated in the human eye, the bond between protein and retinal is broken. To make the receptor sensitive to light again, it must first be regenerated." This occurs within a single layer of cells in the retina, the retinal pigment epithelium. There the protein is reunited with a retinal molecule to form a functional light receptor. "Our light receptors can only be used once and then have to be regenerated in an elaborate process – it’s quite a complicated system."
In the light receptors of cephalopods, insects and many other invertebrates, however, the retinal remains permanently bound to the protein. By absorbing a second light beam, the receptor converts itself back to its original state – and can then receive the next light beam right away. Rhodopsins of this type – termed bistable – can be switched on and off again and again. If the protein could then be modified in such a way that it is switched on with a blue light and switched off with a red light, for example, it would provide the ideal switch.
The secret of the jumping spider
Up until now, bistable light receptors have received little attention from science, but they are the focus of this research project. The bistable rhodopsin from the jumping spider Hasarius adansoni, which reacts to the colour green, has proven to be a particularly promising candidate for the laboratory work. The spider, which is only six millimetres in size, is commonly found in greenhouses all over the world. Of its eight eyes, two large ones point straight ahead. Four superimposed retinal levels in the front eyes allow the spider to precisely pinpoint the location of prey, to within a few millimetres, and to catch it while jumping.
"In contrast to many other rhodopsins, the jumping spider’s rhodopsin is stable and easy to crystallise and manipulate," says Schertler. The researchers hope this protein will spur on the search for light-controlled molecular switches. Looking long-term, scientists consider light-sensitive human melanopsin to be particularly well suited. It regulates our day-night rhythm in special nerve cells in the eye, and it too is bistable. However, no one has yet succeeded in deciphering the structure of melanopsin because it is too unstable in laboratory vessels.
Light in sight
Sonja Kleinlogel of the University of Bern has already produced a chimeric bistable optogenetic protein: the head consists of the light antenna of melanopsin, and the body consists of a receptor found in the bipolar cells of the eye. These play an important role in relaying the signal from the retina to the brain. Using an optogenetics-based gene therapy on blind mice, the scientist managed to restore a large part of their eyesight. However, she constructed her optogenetic protein mainly through trial and error. Thus her method is not currently transferable to other receptors.
"We have to find out why Sonja Kleinlogel’s construct works," says Gebhard Schertler. "And we ourselves need to acquire the knowledge of how we can modify proteins so that they do what we want." Using the Swiss Light Source SLS, SwissFEL, and cryo-electron microscopy (see article “Cool Newcomer” starting on page 18), the researchers want to investigate bistable light receptors and their mechanisms in the cell. Then, on the basis of these findings, they hope to develop additional prototypes for optogenetic tools.
The potential is enormous: such light-controllable switches could be used, for example, to probe higher brain functions. "Classic optogenetics changes the ion balance in nerve cells – we, on the other hand, could activate signal cascades in the brain, which is something completely new," explains Schertler. The technology could one day enable better understanding of mental disorders such as depression and schizophrenia, and perhaps even facilitate the development of new drugs for these diseases.
If G-protein-coupled receptors in the body can be selectively switched on and off, this would also reveal which specific functions a particular receptor has. In drug development, the new technology could be used to check which receptors mediate the effect of a new active ingredient. Notably, this could minimise side-effects.
The development of new light-controlled molecular switches is highly valued by the European Research Council (ERC): in 2020 it awarded a grant of 10 million euros to the European joint project of Gebhard Schertler and his team.
Once the foundations have been laid, this technology will conquer the world of science, Schertler firmly believes. "We are still quite a long way from a toolbox of the kind that already exists for classic optogenetics. But in a few years we will be able to solve a lot of mysteries – and I think hundreds of laboratories will start using such universal light-sensitive GPCR switches."